RESEARCH
Overview:
The Greer Lab is broadly interested in the fundamental questions of how animals sense and interpret exteroceptive and interoceptive chemical signals to generate appropriate organismal responses and how these processes are disrupted in human neurological disorders. To address these questions we are exploring novel mechanisms by which the olfactory system senses behaviorally relevant external stimuli and characterizing the function of microglia in detecting internal chemical cues.
Novel Olfactory mechanisms:
The olfactory system is the principal sensory modality through which most animals extract information about their external surroundings. As a result, considerable effort has gone into understanding how olfactory stimuli are detected and processed to generate appropriate behavioral responses. However, despite a voluminous body of work, progress in understanding how odor sensation couples to behavior has been limited, owing in large part to the immense complexity of the olfactory system. In the mouse there are at least 1200 unique peripheral olfactory sensory neuron (OSN) subtypes, each of which is stimulated by distinct, but partially overlapping, sets of odorants, making it challenging to gain insight into how a given chemical triggers a specific behavioral output.
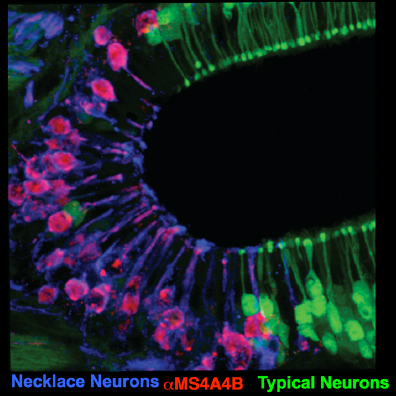
To attempt to overcome the limitations associated with studying such a heterogeneous collection of peripheral sensory neurons, we are focusing our efforts on a population of OSNs, known as necklace cells, which despite representing a single OSN subtype, nonetheless constitute ~5% of the OSNs in the mouse. Necklace cells detect a number of ethologically relevant chemical compounds, including predator odorants, pheromones, and noxious gasses, suggesting that the necklace system is important for mediating behaviors critical to animal survival. Despite this evidence suggesting a crucial role for the necklace olfactory subsystem, a clear picture of the biological role of the necklace is lacking in large part because the mechanism by which these cells sense odorants and transmit this information to higher cognitive regions was unknown.
To begin to bridge this critical gap in knowledge, we recently discovered that necklace cells express a new family of mammalian olfactory chemoreceptor, the Membrane Spanning 4A (MS4A) proteins (Greer et al., Cell, 2016). Surprisingly, unlike all previously identified mammalian odorant receptors (ORs), MS4A proteins do not belong to the G protein-coupled receptor (GPCR) superfamily and are not expressed in a one-receptor-one-neuron pattern. Rather, each Ms4a gene encodes a four-pass transmembrane protein, and individual mouse OSNs co-express many MS4A receptors. Despite their lack of similarity with known mammalian ORs in either structure or expression logic, the MS4As are necessary and sufficient to allow necklace OSNs to sense a number of ethologically relevant odorants, including predator-derived chemicals, pheromones, and dietary fatty acids. These results suggest that the necklace olfactory subsystem is a powerful platform for investigating the neural mechanisms by which particular olfactory stimuli couple to behaviors that are vital for survival.
In addition to our work in the necklace system, we have also identified novel populations of olfactory sensory neurons and we are investigating that types of chemicals these cells detect, where within the brain they transmit information, and ultimately what sorts of behaviors they have evolved to mediate. Together, our goal is to use the mammalian olfactory system as a model for understanding how animals respond appropriately to ethologically relevant chemical stimuli.
Microglia:
In parallel to our work in the olfactory system, we have also begun to explore how interoceptive chemical cues are detected by microglia, the resident immune cells of the nervous system. Microglia represent 10% of all of the cells in the brain and have been implicated in a variety of nervous system processes ranging from immune responses to pathogens to synaptic development and refinement. In addition to their role in physiological settings, microglia are also thought to play a critical role in a variety of devastating human neurological disorders including Parkinson’s Disease, ALS, and Alzheimer’s Disease.
Our interest in microglia originally stemmed from our observation that a subset of MS4As is highly expressed in microglia where they facilitate the detection of previously identified MS4A ligands. Intriguingly, many recent human genome wide association studies (GWAS) have strongly linked Ms4a mutations with Alzheimer’s Disease (AD), a disease in which microglia are thought to play a crucial, but unclear, role. Based on these observations, we plan to elucidate the function of MS4As in microglia in the hope that this will both expand our knowledge of MS4A chemoreceptors and microglia and provide insight into a debilitating human cognitive disorder for which there are limited therapeutic interventions.